Rutinosidases and hesperidinases (6-O-α-rhamnosyl-β-glucosidases) are unique microbial disaccharide-specific glycosidases (diglycosidases) that recognize the heterosidic bond between a disaccharide and its aglycone. Their transglycosylation activities can be exploited for one-step synthesis of a wide range of non-natural diglycosides with different functional groups. The first gene for a rutinosidase (Aspergillus niger, CAZY GH5) was cloned (Šimčíková 2015) and crystallized (Pachl 2020). In a joint project with the laboratory of Prof. J. Breccia (National University of La Pampa, Argentina), we identified the first rutinosidase-encoding gene from a prokaryote (Actinoplanes missouriensis, CAZY GH55) (https://link.springer.com/article/10.1007/s00253-015-7088-x). Acremonium sp. was found to encode two rutinosidases with largely different substrate specificities, one accepting only 7-O-β-rutinosides (hesperidin, CAZY GH5) and the other also accepting 3-O-β-rutinosides (e.g., rutin or narcissin, CAZY GH3) (Weiz 2019).
The recombinant enzyme, which proved to have outstanding synthetic capabilities was used in transglycosylation reactions transferring rutinose from rutin as a glycosyl donor to various primary (saturated and unsaturated), secondary, acyclic, and phenolic alcohols (Fig. 1). Several naturally occurring glucosides were enzymatically synthesized in one-pot telescoping reactions in collaboration with Dr. Sergio Riva’s laboratory (CNR, Milano, IT), using rutinosidase from A. niger and a rhamnosidase from A. terreus for trimming the rhamnosyl moiety from the rutinose-based products (Bassanini 2017). Rutinosidase from A. niger is capable of glycosylating some carboxylic acids (e.g., coumaric, ferulic, and caffeic acids) as acceptors. Surprisingly, rutinosylation occurred both at the phenolic hydroxyl group of the acceptor and at the carboxyl group, which is an entirely unprecedented finding in glycosidases (Fig. 3) (Bassanini 2019).
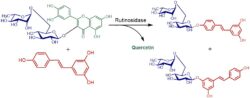
Example of transglycosylation of resveratrol catalyzed with rutinosidase (Šimčíková 2015).
Glycosylation of inorganic azide with non-mutated glycosydase is another unique feature of rutinosidase (A. niger). This reacion affords b-rutinosyl azide (Fig. 2B), whereas catalytic nucleophile mutant of this enzyme yields α-rutinosyl azide – both reaction reactions on a preparative. When quercetin-b-glucopyranoside is used as a glycosyl donor corresponding b-glucopyranosyl azides are formed (Kotik 2021).
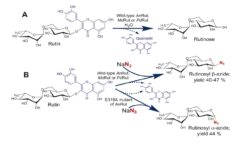
Hydrolytic reaction of rutin catalyzed by rutinosidases from A. niger, Penicillium chrysogenum and Mucor circinelloides (A); glycosylation of azide with wild type rutinosidases and mutated rutinosidase (B) (Kotik 2021).
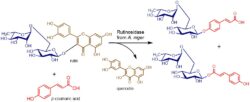
Glycosylation of carboxylic moiety of the p-coumaric acid with rutinosidase (Bassanini 2019).
A novel concept of enzymatic hydrolysis with extremely high concentrations of rutin was developed in our laboratory and published recently (Kapešová 2019). The reaction mixtures contained rutin at concentrations up to 300 g L-1 (corresponding to ~0.5 M), and the reactions were carried out in the absence of any organic solvents using the recombinant rutinosidase from A. niger as catalyst (Fig. 2A). Full conversions were achieved, although rutin and the hydrolytic product quercetin were largely in the undissolved state. We referred to these biotransformations as „solid-state conversions.“
Projects


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.