Flavonolignans silybin (1) and 2,3-dehydrosilybin (2) are biologically important polyphenols [] with radical .
Molecular mechanisms of their antiradical activity were described by us quite recently, using selectively methylated derivatives of 1 and 2 (Figure 1), which afforded after reaction with radicals (e.g. DPPH) or enzyme laccase dimers [,3]. Structure determination (NMR, MS) of these products enabled us to elucidate reaction mechanism after radical attack.
The results were further supported by evaluation of antioxidant activity of these compounds, e.g. DPPH scavenging assay, inhibition of lipid peroxidation, and by quantum-chemical calculations [].
Figure 1. Structures of the studied compounds
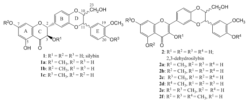
Table 1. Antioxidant activities of the studied compounds
| Compound | DPPH | LPx |
|---|---|---|
| IC50 (μM) | IC50 (μM) | |
| 1 | 1745 | 73.92 |
| 1a | 2541 | 33.69 |
| 1b | 5750 | 25.87 |
| 1c | 3546 | 89.73 |
| 2 | 54 | 15.11 |
| 2a | 680 | 24.64 |
| 2b | 292 | 93.30 |
| 2c | 66 | 26.54 |
| 2d | 60 | 13.41 |
LPx – lipid peroxidation inhibition
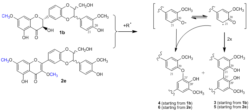
Unmodified silybin (1) and 2,3-dehydrosilybin (2) gave in reactions with radicals products of polymeric nature, whose structural characterization is not feasible. Selective methylation of suitable positions of their molecules terminates the radical reactions at the stage of dimers, which can be isolated and structurally characterized [].
Mechanisms of radical reactions leading to dimeric products
Scheme 1. Mechanism of the formation of C20-O-C21‘ and C21-C21‘ dimers in both silybin and 2,3-dehydrosilybin derivatives with “free“ 20-OH group
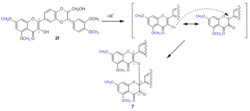
Scheme 2. Mechanism of the formation of C2‘-O-C3 dimer in 2,3-dehydrosilybin derivatives with 3-OH group
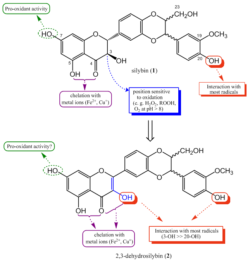
The knowledge of active sites of silybin and 2,3-dehydrosilybin
Scheme 3. The role of silybin and 2,3-dehydrosilybin hydroxyl groups in mechanism of their antioxidant activity
Collaboration
- Institute of Microbiology, Academy of Sciences of the Czech Republic Laboratory of Molecular Structure Characterization, Vídeňská 1083, CZ-142 20 Prague, Czech Republic
- Dr. Petr Sedmera – structure characterization (NMR)
- Institute of Physiology, Academy of Sciences of the Czech Republic, Vídeňská 1083, CZ-142 20 Prague, Czech Republic
- Dr. Marek Vrbacký, Dr. Zdeněk Drahota – Experiments with brown adipose tissue mitochondria
- Institute of Medical Chemistry and Biochemistry, Faculty of Medicine, Palacký University, Hněvotínská 3, CZ-775 15 Olomouc, Czech Republic
- Doc. Jitka Vostálová, Doc. Daniela Walterová – Antioxidant assays (DPPH, inhibition of lipid peroxidation, superoxide scavenging, cyclic voltammetry)
- Laboratoire de Biophysique, Université de Limoges, 2 rue du Docteur Marcland, 87025 Limoges, France
- Dr. Patrick Trouillas – Quantum-chemical calculations
Financial support
This work was supported by grant KJB400200701 of the Grant Agency of the Academy of Sciences of Czech Republic, grants 303/06/1261 and 303/08/0658 of the Grant Agency of the Czech Republic, MSMT grant ME 922 and by Institutional research concepts AV0Z50200510 and MSM 6198959516.
References
- Gažák R, Walterová D, Křen V. Silybin and silymarin – new and emerging applications in medicine. Curr Med Chem 2007;14:315–338
- Gažák R, Sedmera P, Vrbacký M, Vostálová J, Drahota Z, Marhol P, Walterová D, Křen V. Molecular mechanisms of silybin and 2,3-dehydrosilybin antiradical activity- role of individual hydroxyl groups. Free Radic Biol Med 2009;46:745–758
- Gažák R, Sedmera P, Marzorati M, Riva S, Křen V. Laccase-mediated dimerization of the flavonolignan silybin. J Mol Catal B-Enzym 2008;50:87–92
- Trouillas P, Marsal P, Svobodová A, Vostálová J, Hrbáč J, Gažák R, Křen V, Lazzaroni R, Duroux J-L, Sedmera P, Walterová D. Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin flavonolignans: A joint experimental and theoretical study. J Phys Chem A 2008;112:1054–1063


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.