Flavonolignans, flavonoids and other phenolic compounds are frequently present in nutraceuticals and have intriguing biological properties. The process of their metabolism, as well as the metabolites formed and their biological effects, are subjects of avid interest among scientists.
In Phase II of biotransformation, phenolic compounds form conjugates such as methyl ethers, sulfates or glucuronides (Figure I). Several methods of enzymatic sulfation were therefore developed and employed with various flavonolignans, flavonoids and simpler polyphenols. Aryl sulfatase from rat liver was used in sulfation of silybin (Purchartová, 2013), quercetin and taxifolin (Purchartová, 2015). Another, more robust method employed aryl-sulfate sulfotransferase from Desulfitobacterium hafniense in combination with a sulfate donor, allowing for sulfation of multiple flavonoids and flavonolignans (Marhol, 2013; Purchartová, 2015, Valentová, 2017, Valentová 2018, Káňová, 2020), and also for the sulfation of small phenolic compounds which are potential flavonoid metabolites (Begines, 2019; Petrásková, 2022). In case of glucuronidated metabolites, Streptomyces catalyzed glucuronidation was employed to obtain glucuronides of silybin A and B (Charrier, 2014). The synthesized compounds (sulfates and glucuronides) serve as standards in metabolic studies and studies evaluating their biological activity.
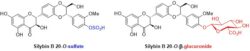
Figure I: Examples of flavonolignan metabolites
The biological effects of flavonolignan and flavonoid metabolites, as well as the metabolic processes involving flavonolignans and flavonoids, were studied in cooperation with other institutions, mainly the Department of Medical Chemistry and Biochemistry of the Palacký University, but also with the Department of Food Science of the Czech University of Life Sciences, the Department of Biochemistry and Microbiology of the University of Chemistry and Technology, the Department of Pharmacology and Toxicology of the Charles University, and other institutions. A comprehensive in vitro study of hepatic biotransformation of eight flavonolignans identified multiple flavonolignan metabolites and confirmed that the metabolic processes in hepatocytes are conjugation reactions including glucuronidation, sulfation and methylation (Vrba, 2018). Another study examined the metabolism of flavonolignans by intestinal microbiota (Valentová, 2020). In an in vitro study, specific UDP-glucuronosyltransferases potentially involved in glucuronidation of six flavonolignans by human liver microsomes were identified (Vrba, 2020). Similarly, by examining the interaction of six flavonolignans with SULT enzymes, with human liver and intestinal cytosols, and also with human hepatocytes, several potential enzymes involved in the hepatic or intestinal sulfation of flavonolignans in humans were also determined (Vrba, 2020). The study of their interactions with human hepatocytes was later carried out for two additional flavonolignans (Vrba, 2021).
For a long time, antiradical properties were considered to be the source of various beneficial effects of polyphenol-rich diet, and as such, these properties of sulfated phenolic metabolites were examined in multiple studies (Roubalová, 2015, Valentová, 2017). Meanwhile, a more recent study pointed out the low effect of sulfated flavonoid metabolites on the nuclear erythroid 2-related factor 2 (Nrf2) (Valentová 2018), while another study demonstrated that monosulfates of several flavonolignans displayed vasorelaxant activity ex vivo (Pourová, 2019). Interactions of flavonolignans and their sulfated metabolites with human serum albumin and cytochrome P450 enzymes were examined, showing that the sulfated metabolites of flavonoligans form stable complexes with serum albumin and inhibit the tested cytochrome P450 enzymes, with similar affinities as their parent compounds (Faisal, 2021).


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.