In nature, silybin occurs as an approximately equimolar pair of diastereomers – silybin A and silybin B. Since the diastereomers exhibit different biological activities, their separation is necessary. C18 HPLC is prohibitively laborious and suitable only for minuscule amounts. Chemoenzymatic flow chemistry approach was therefore developed (Gažák 2010, Monti 2010).
Preparatory separation of silybin
Preparatory separation of silybin A/B pair is based on the ability of lipase from Candida antarctica (yeast) to transesterify primary alcoholic group of silybin. Silybin B is transesterified in higher rate, thus allowing the separation.

Transesterification of silybin A/B with Candida antarctica lipase.
Candida antarctica lipase; pdb: 1TCA
The flow chemistry setup
The reactions were performed in a flow setup (Biedermann 2021). The double jacketed, thermostated column was filled with catalyst immobilized on polyurethane beads. Reactants were injected into the column and pumped through. The products were collected after the passage and separated.

Actual flow chemistry setup.
Block scheme of the flow chemistry setup.
Exploitation of Novozym 435 for the preparation of silybin A and silybin B
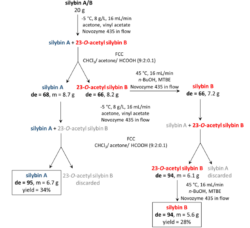
Practical separation of silybin diastereomers proceeds in multiple steps as shown below. The diastereomeric resolution in flow is alternated with flash column chromatography (FCC). Diastereomeric excess (de) is a measure of optical purity of the diastereomers.


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.