The bioavailability and therapeutic efficacy of silybin is rather limited by its very low solubility in water (430 mg/L). A number of silybin water-soluble semisynthetic derivatives were prepared [], however, an increase in their water-solubility usually led to an impairment of their antioxidant (antiradical) activity in comparison with silybin [4].
On the contrary, non-covalent complex of silybin with phosphatidyl choline – (Silipide, IdB 1016, Indena, IT) (…., Buzzelli 1993), possessed better bioavailability and higher antioxidant activities (Carini 1992, Comoglio 1995) than silybin (Morazzoni 1992, Barzaghi 1990). Moreover, Silipide also exhibits promising anticancer activities (Gallo 2003).
Lipophilization of silybin possibly improves its membrane-stabilizing function – one of the basic molecular mechanisms of silybin activity (Valenzuela 1994, Lettéron 1990). An improvement of the antioxidant activity in heterogeneous systems by lipophilization of the hydrophilic antioxidant has previously been observed: the antioxidant efficiency of retinyl ascorbate (Abdulmajed 2005) as well as ferulates, dihydroferulates, caffeates, dihydrocaffeates and gallates were generally better antioxidants than the parent acids (Hunneche 2008, Lu 2006, Kikuzaki 2002).
Scheme 1. Reagents and conditions
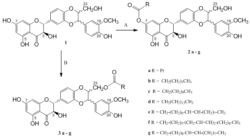
- A. Acyl chloride (1.5 equiv.), Py, 0 °C, 1 h (29–45 %);
- B. Acyl chloride (1 equiv.), BF3•Et2O (2.4 equiv.), CH2Cl2/MeCN (1:1, v/v), 2 h at 0 °C, then 1 h at r.t. (28–32 %).
Table 1. Antioxidant anti-influenza virus A/PR8/34/ (H1N1) activity of silybin and its fatty acid esters
| Compound | Antioxidant activity | Antiviral activity |
|---|---|---|
| LPX (a) | EC50 (b) (µmol/L) | |
| IC50 (μmol/L) | ND | |
| Silybin (1) | 58.1 | 14 |
| 7-O-Butanoylsilybin (2a) | 100.6 | 6 |
| 7-O-Octanoylsilybin (2b) | 116.7 | 6 |
| 7-O-Dodecanoylsilybin (2c) | 44.7 | ND (c) |
| 7-O-Palmitoylsilybin (2d) | 28.9 | 24 |
| 23-O-Butanoylsilybin (3a) | 91.7 | 18 |
| 23-O-Octanoylsilybin (3b) | 61.1 | 3 |
| 23-O-Dodecanoylsilybin (3c) | 50.5 | 3 |
| 23-O-Palmitoylsilybin (3d) | 51.9 |
- (a) LPX – Inhibition of microsomal lipid peroxidation – the activity was calculated as the concentration of the tested compound inhibiting the color reaction with thiobarbiturate by 50 % (IC_50).
- (b) EC50 represents the concentration of compound required to reduce plaque number by 50% relative to the control well without test compound.
- © CC50 and EC50 of 2d were not determined due to its low water solubility.
Conclusion
Two selective acylation methods for silybin esterification with long-chain fatty acids were developed, yielding a series of silybin 7-O- and 23-O-acyl-derivatives of varying acyl chain lengths. These compounds were tested for their (inhibition of lipid peroxidation and DPPH-scavenging) and anti-influenza virus activities. The acyl chain length is an important prerequisite for both biological activities, as they improved with increasing length of the acyl moiety (Gažák 2010).
Collaboration
- Institute of Microbiology, Academy of Sciences of the Czech Republic Laboratory of Molecular Structure Characterization, Vídeňská 1083, CZ-142 20 Prague, Czech Republic
- Dr. Petr Sedmera – structure characterization (NMR)
- Institute of Medical Chemistry and Biochemistry, Faculty of Medicine, Palacký University, Hněvotínská 3, CZ-775 15 Olomouc, Czech Republic
- Dr. Kateřina Valentová – Antioxidant assays (inhibition of lipid peroxidation)
- The Institute of Scientific and Industrial Research, Department of Organic Fine Chemicals, Osaka University, 8–1 Mihogaoka, Ibaraki, Osaka, 567–0047, Japan
- Dr. Nobuo Kato, Dr. Hiroyo Matsumura, Dr. Kunihiro Kaihatsu (Evaluation of antiviral activities of silybin esters)
Financial support
This work was supported by grant KJB400200701 from the Grant Agency of the Academy of Sciences of Czech Republic and by institutional research concept AV0Z50200510 and grants OC08049, LC06010 and MSM6198959216 from the Ministry of Education, Youth and Sports of the Czech Republic.
References
- R. Braatz, K. Gurler, G. Bergish, G. Halbach, H. Soicke, K. Schmidt, Czech. Patent 273 610, 1985; Chem. Abstr. 105 (1985) P127476b
- G. Pifferi, R. Pace, M. Conti, Il Farmaco 49 (1994) 75–76
- V. Křen, J. Kubisch, P. Sedmera, P. Halada, V. Přikrylová, A. Jegorov, L. Cvak, R. Gebhardt, J. Ulrichová, V. Šimánek, J. Chem. Soc., Perkin Trans. 1 (1997) 2467–2474
- R. Gažák, A. Svobodová, J. Psotová, P. Sedmera, V. Přikrylová, D. Walterová, V. Křen, Bioorg. Med. Chem. 12 (2004) 5677–5687
- M. Conti, S. Malandrino, M.J. Magistretti, Jpn. J. Pharmacol. 60 (1992) 315–321
- G. Buzzelli, S. Moscarella, A. Gusti, A. Duchini, C. Marena, M. Lampertico, Int. J. Clin. Pharmacol. Ther. Toxicol. 31 (1993) 456–460
- R. Carini, A. Comoglio, E. Albano, G. Poli, Biochem. Pharmacol. 43 (1992) 2111–2115
- A. Comoglio, A. Tomasi, S. Malandrino, G. Poli, E. Albano, Biochem. Pharmacol. 50 (1995) 1313–1316
- P. Morazzoni, M.J. Magistretti, C. Giachetti, G. Zanolo, Eur. J. Drug. Metab. Pharmokinet. 17 (1992) 39–44
- N. Barzaghi, F. Crema, G. Gatti, G. Pifferi, E. Perucca, Eur. J. Drug Metab. Pharmokinet. 15 (1990) 333–338
- D. Gallo, S. Giacomelli, C. Ferlini, G. Raspaglio, P. Apollonio, S. Prisley, A. Riva, P. Morazzoni, E. Bombardelli, G. Scambia, Eur. J. Cancer 39 (2003) 2403–2410
- A. Valenzuela, A. Garrido, Biol. Res. 27 (1994) 105–112
- P. Letteron, G. Labbe, C. Degott, A. Berson, B. Fromenty, M. Delaforge, D. Larrey, D. Pessayre, Biochem. Pharmacol. 39 (1990) 2027–2034


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.