Silybin was reported to act synergistically with chemotherapeutics (e.g. paclitaxel, cisplatin) resulting in the induction of apoptosis(Pashaei-Asl 2018). One of the possible explanations for this effect is the inhibition of efflux transporters responsible for multidrug resistance (MDR) (Maitrejean 2000).
In 2006 we reported a SAR study of the effect of O-substitution on the cytotoxicity and P-glycoprotein modulatory activity of silybin and dehydrosilybin. Alkylation at 7-OH increased cytotoxicity of dehydrosilybin derivatives. Derivatives with modification on E-ring showed inhibitory activity at low concentrations (Džubák 2006).
We reported that silychristin A and its derivatives (dehydrosilychristin, anhydrosilychristin and isosilychristin, Figure 1) inhibited P-glycoprotein (P-gp) in a concentration-dependent manner, sensitizing the multidrug-resistant ovarian cancer cell line (HOC/ADR, A2780/ADR) to doxorubicin (Viktorová 2019).
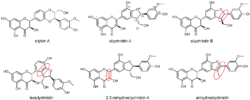
Figure 1. Structures of silychristin derivatives tested.[4]
In 2022 we reported that 2,3-dehydrosilybin B reverts antibiotic resistance of Staphylococcus aureus. We evaluated that 2,3-dehydrosilybin B acts as a non-competitive inhibitor of NorA and MepA efflux pumps and it decreases antibiotic-induced expression of efflux pumps (Holasová 2022).
Collaboration: Dr. Jitka Viktorová – modulation of multidrug resistance, University of Chemistry and Technology Prague, Technická 5, Prague 166 28.
Financial support
This work was supported by Czech Science Foundation (project No. 21-00551S) and by the Ministry of Education, Youth and Sports of the Czech Republic (mobility project LTC20070).
Reviews for further reading:
Chambers, C. S., et al. J. Agric. Food Chem. 68, 1763-1779 (2020)
Křen, V. Int. J. Mol. Sci. 22 (2021)


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.