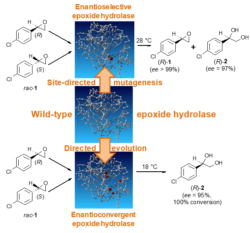
Single-enantiomer pharmaceuticals are a growing market with an extensive number of chiral intermediates that are needed for their synthesis. Enantiopure epoxides and vicinal diols are versatile building blocks for the synthesis of various biologically active compounds. They can be obtained from inexpensive racemic epoxides using some wild-type or engineered epoxide hydrolases by kinetic resolution or enantioconvergent hydrolysis. Especially interesting are enantioconvergent epoxide hydrolases because in enantioconvergent processes there is no 50% yield limitation, which is linked to any resolution process.
Further reading
- Kotik M, Štěpánek V, Grulich M, Kyslík P, Archelas A. Access to enantiopure aromatic epoxides and diols using epoxide hydrolases derived from total biofilter DNA. Journal of Molecular Catalysis B-Enzymatic, 65 (2010) 41−48.
- Kotik M, Archelas A, Faměrová V, Oubrechtová P, Křen V. Laboratory evolution of an epoxide hydrolase – Towards an enantioconvergent biocatalyst. Journal of Biotechnology, 156 (2011) 1−10.
- Kotik M, Zhao W, Iacazio G, Archelas A. Directed evolution of metagenome-derived epoxide hydrolase for improved enantioselectivity and enantioconvergence. Journal of Molecular Catalysis B-Enzymatic, 91 (2013) 44−51.
- Kotik M, Zhao W, Iacazio G, Archelas A. Enantioselective bio-hydrolysis of various racemic and meso aromatic epoxides using the recombinant epoxide hydrolase Kau2. Advanced Synthesis and Catalysis, 357 (2015) 1895−1908.
- Kotik M, Archelas A, Wohlgemuth R. Epoxide hydrolases and their application in organic synthesis. Current Organic Chemistry, 16 (2012) 451−482.


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.