( L. Martínková, M. Chmátal, V. Křen, M. Pátek, L.Rucká, J. Nešvera)
Nitrile hydrolysis is an important step in the synthesis of carboxylic acids and amides (building
blocks for the synthesis of fine and pharmaceutical chemicals). It has been traditionally carried
out under extreme conditions (strong acids or bases, high temperatures). The disadvantages of this
method are high energy costs, low yields and missing selectivities.
However, the enzymes of the nitrile metabolism (Figure 1) perform these reactions under mild
conditions, enantio- or regioselectively and with high yields. These enzymes consist of nitrilases
and nitrile hydratases which transform nitriles into carboxylic acids and amides, respectively.
Amides are then hydrolyzed by amidases. The synthesis of nitriles from aldoximes is catalyzed by
aldoxime dehydratases. *
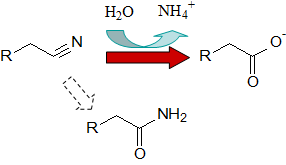
Figure 1: Reactions catalyzed by enzymes of nitrile metabolism
The focus of our work is on the acquisition of new representatives of the aforementioned enzymes,
e.g. by mining gene databases (GenBank) and expressing the selected, artificially synthesized genes
in . In this way a set of more than 15 nitrilases from filamentous fungi was constructed. These
catalysts (cells, purified enzymes) were used in the synthesis of alfa-hydroxy acids (building
blocks, cosmetics) or new derivatives of taxol (anticancer drug) from the corresponding
nitriles.
Some bacterial nitrilases were prepared analogously and proved useful in the biotransformation of
benzonitrile herbicide residues into less toxic and biodegradable carboxylic acids (Figure 2).

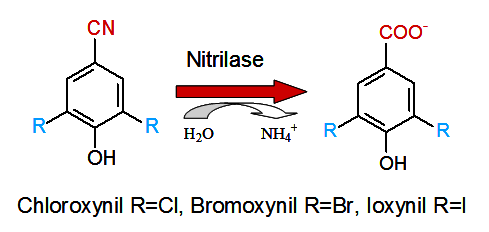
Figure 2: Enzymatic detoxication of benzonitrile herbicides
Literature
- Veselá AB, Pelantová H, Šulc M, Macková M, Lovecká P, Thimová M, Pasquarelli F,
Pičmanová M, Pátek M, Bhalla TC, Martínková L. (2012) Biotransformation of benzonitrile
herbicides via the nitrile hydratase-amidase pathway in rhodococci. J Ind Microbiol Biotechnol
39:1811–1819 - Rucká L, Volková O, Pavlík A, Kaplan O, Kracík M, Nešvera J, Martínková L, Pátek M
(2014) Expression control of nitrile hydratase and amidase genes in Rhodococcus erythropolis and
substrate specificities of the enzymes. Antonie van Leeuwenhoek 105:1179–1190 - Veselá AB, Křenková A, Martínková L (2015) Exploring the potential of fungal
arylacetonitrilases in mandelic acid synthesis. Mol Biotechnol 57:466–474 - Wilding B, Veselá AB, Perry JJB, Black GW, Zhang M, Martínková L, Klempier N (2015) An
investigation of nitrile transforming enzymes in the chemo-enzymatic synthesis of the taxol
sidechain. Org Biomol Chem 13:7803–7812 - Veselá AB, Rucká L, Kaplan O, Pelantová H, Nešvera J, Pátek M, Martínková L. (2016)
Bringing nitrilase sequences from databases to life: the search for novel substrate specificities
with a focus on dinitriles. Appl Microbiol Biotechnol 100:2193–2202 - Martínková L, Rucká L, Nešvera J, Pátek M (2017) Recent advances and challenges in the
heterologous production of microbial nitrilases for biocatalytic applications. World J Microbiol
Biotechnol 33:art.no. 8
Cooperation
- P. Lovecká et al., University of Chemical Technology, Prague
- N. Klempier, TU Graz, Austria
- M. Cantarella, University of L´Aquila, Italy
- T.C. Bhalla, University of Himachal-Pradesh, India
- CMST-Action CM1303, Systems Biocatalysis – SysBiocat


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.