By definition, glycosidases (glycoside hydrolases; EC 3.2.1) are hydrolases that naturally catalyze the cleavage of glycosidic bonds, either between two or more carbohydrate units or between a carbohydrate and its aglycone moiety. Advantageously, under certain conditions many retaining glycosidases are able to catalyze the synthesis of glycosidic bonds. In this kinetically controlled process called transglycosylation, the carbohydrate moiety is transferred from an activated donor to a free hydroxyl group of acceptors such as alcohols or carbohydrates, instead of water. Unfavorably for synthetic applications, the yields of transglycosylation reactions are substantially decreased by the concurrent hydrolysis of the donor substrate as well as of the synthesized product. At this point, enzyme engineering aimed at improving the synthetic capabilities of glycosidases comes into play. By impairing their hydrolytic activity while retaining their transglycosylation activity, novel synthetic engines arise that may change the future of the biosynthesis of tailored complex carbohydrates and glycoconjugates.
Our group is focused mainly on the enzyme engineering and structure-activity relationship studies of fungal b-N-acetylhexosaminidases capable of the synthesis of bioactive chitooligomers with a wide range of degree of polymerization and their derivatives suitable for conjugation to other structures (Fig. 1).
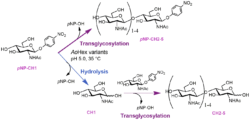
Figure 1. Complex transglycosylation reactions catalyzed by mutant variants of AoHex (Mészáros 2022)
Our next goal regarding β-N-acetylhexosaminidases is to modulate the substrate specificity of β-N-acetylhexosaminidase. As it is an enzyme with dual substrate specificity, towards GlcNAc and GalNAc. By mutagenesis in/around the active site, we can shift the enzyme activity in favor of GlcNAcase or GalNAcase activity, or influence transglycosylation and hydrolytic activity.

Figure 2. Model of the active site of β-N-acetylhexosaminidase from T. flavus with docked GlcNAc-thiazoline, the reaction intermediate. Scheme outline possible mutant variants of this enzyme. (Mészáros 2021, Slámová 2015, Kapešová 2020, Nevasilová 2020, Nekvasilová 2022)
The other major goal is the glycosidase-catalyzed synthesis of human milk oligosaccharides (HMOs) and their analogs, which could be used in infant formulas and food supplements, employing both wild-type and engineered glycosidases (Fig. 3).
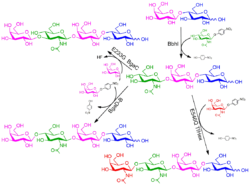
Figure 3. Enzymatic synthesis of HMO analogs (Nekvasilová 2022)
We aim to synthesize the HMO backbone using beta-N-acetylhexosaminidases, beta-galactosidases and/or glycosyltransferases. The base chain can be further decorated with fucose or sialic acid. We focus on the fucosylation of the HMO backbone using alpha-fucosidases, the substrate specificity of alpha-fucosidases and the production of new mutant variants to increase the transglycosylation potential.

Figure 4. Scheme of the transfucosylation reaction catalyzed by alpha-fucosidase, fucosynthase and fucosyltransferase
For the galactosylation of oligosaccharides, we primarily use β-galactosidase from Bacillus circulans (BgaD), which is distinguished by its high transglycosylation activity. The enzyme exists in four isoforms—A (189 kDa), B (155 kDa), C (132 kDa), and D (92 kDa)—produced through endogenous proteolytic activity. Among these, isoform A (BgaD-A) is particularly notable for its distinct synthetic capabilities. In our lab, we have produced isoforms A, B, and D. We investigated the synthetic potential of BgaD-A through site-directed mutagenesis, constructing three mutants at the active-site catalytic nucleophile E532 as potential glycosynthases. However, none of these mutants were capable of processing α-galactosyl fluoride as a galactosyl donor (Hovorková, ChemCatChem, 2021). The structural differences between the longest (A) and the shortest (D) isoforms were further investigated using Cryo-EM. A newly identified „Barrier Domain 8“ was found to act as a barricade, obstructing the access of longer oligosaccharide substrates into the active site of BgaD-A (Hovorková, Kaščáková, submitted 2024).

Recent publications:
Mészáros, Adv. Synth. Catal. 2022
Nekvasilová, Int. J. Mol. Sci 2022
Mészáros, Biotechnol. Adv. 2021
Nekvasilová, Adv. Synth. Catal. 2020
Kapešová, Int. J. Biol. Macromol. 2020


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.