Various hybrid molecules aimed at improving the bioactivities of flavonoids and to study redox interactions were synthesized in the laboratory and their properties investigated. Isoquercitrin esters with mono- or dicarboxylic or aromatic acids and their homologues (Vavříková 2016, Heřmánková-Vavříková 2017) were efficient inhibitors of lipid peroxidation with increased lipophilicity.
Selective galloylation at C7-OH increased the effect of 2,3-dehydrosilybin on human umbilical vein endothelial cells (Karas 2016, Pivodová 2016, Karas 2017).
We also prepared retinoyl-flavonolignan hybrids with improved antioxidant properties (Chambers 2019).
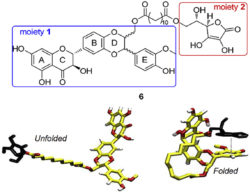
Finally, a series of antioxidants was designed and synthesized based on conjugation of silybin with L-ascorbic acid, trolox alcohol or tyrosol via a C-12 aliphatic linker. The silybin-ascorbic acid conjugate exhibited excellent electron donating ability and displayed the best activities (IC50 = 30 µM) in terms of lipid peroxidation inhibition (Vavříková 2017).


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.