L. Martínková, , M. Kotík, M. Chmátal, V. Křen
Tyrosinases and laccases belong to copper oxidases acting on phenolic substrates. Their ability to oxidize a broad spectrum of these compounds makes them promising for biocatalysis and bioremediation. Tyrosinase is the key enzyme of melanin synthesis in skin and hairs. Therefore, it has been also used in the screening for inhibitors of pigmentation disorders.
Our study of laccases from fungi focused on their use for the biotransformation of brominated flame retardants. In particular, tetrabromobisphenol A (TBBPA) is used in large amounts (over 100,000 tons per year) to improve fire safety of plastics and textiles. Laccases were found to be effective in its degradation. Some of the reaction products (Figure 1) are the same as the mammalian metabolites (Zalko et al. (2006) Chemosphere 64:318–327). Therefore, biotransformations by laccase are suitable to produce these compounds for toxicity studies.
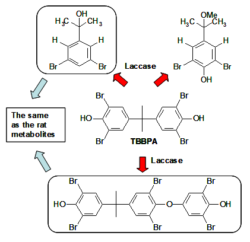
Figure 1: Biotransformation of the flame retardant TBBPA by laccase
A new tyrosinase was prepared by expressing the gene from the Polyporus arcularius fungus in Escherichia coli and used as a model for testing the inhibitory effects of flavonoids such as aurones (yellow plant pigments). These inhibitors have promising applications in the suppression of excessive melanin formation (Figure 2), which occurs in food browning and skin hyperpigmentation.
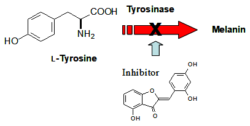
Figure 2: Suppresion of melanogenesis by tyrosinase inhibitors (here: an aurone derivative)
In addition, tyrosinases are powerful tools for the remediation of wastewaters which contain toxic phenols. Using a tyrosinase from the common button mushroom (Agaricus bisporus), phenols (1.5 g L-1) were almost totally degraded in coking effluents. The action of these enzymes on phenols was enabled by the preceding biodegradation of cyanide in these heavily polluted wastewaters (see Remediation of cyanide wastes).
Literature
- B. Uhnáková, R. Ludwig, J. Pěknicová, L. Homolka, L. Lisá, M. Šulc, A. Petříčková, F. Elzeinová, H. Pelantová, D. Monti, V. Křen, D. Haltrich, L. Martínková: Biodegradation of tetrabromobisphenol A by oxidases in basidiomycetous fungi and estrogenic activity of the biotransformation products. Bioresource Technol (2011) 102, 9409–9415.
- Marková E, Kotik M, Křenková A, Man P, Haudecoeur R, Boumendjel A, Hardré R, Mekmouche Y, Courvoisier-Dezord E, Réglier M, Martínková L (2016) Recombinant tyrosinase from Polyporus arcularius: Overproduction in Escherichia coli, characterization, and use in a study of aurones as tyrosinase effectors. J Agric Food Chem 64:2925–2931
- Martínková L, Kotik M, Marková E, Homolka L (2016) Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: A review. Chemosphere 149:373–382
- Martínková L, Chmátal M (2016) The integration of cyanide hydratase and tyrosinase catalysts enables effective degradation of cyanide and phenol in coking wastewaters. Water Res 102:90–95
Cooperation
- Aix-Marseille University, Marseille, France
- Třinec Iron Steel Works, Třinec, Czech Republic


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.