P. Bojarová, K. Slámová, L. Petrásková, A. Drozdová
β-N-Acetylhexosaminidases (EC 3.2.1.52, CAZy GH family 20) are glycosidases featuring dual enzymatic activity, naturally cleaving both β-GlcNAc and β-GalNAc moieties off various glycostructures. β-N-Acetylhexosaminidases from filamentous fungi have recently gained a lot of attention, due to their unique enzyme architecture and mainly their extremely broad substrate specificity and great synthetic potential in the preparation of unnatural oligosaccharides. Extracellular β-N-acetylhexosaminidases from filamentous fungi are able to cleave and transfer substrates bearing various functionalities, ranging from carboxylates, sulphates, acylations to azides, and even 4-deoxy glycosides. Thus, they have proved to be versatile biosynthetic tools for the preparation of both natural and modified hexosaminides under mild conditions with good yields. Oligosaccharides composed of N-acetylhexosaminides carrying a negatively charged moiety have been identified as strong ligands of the activation receptors of natural killer cells, particularly of CD69 protein, which suggests application in cancer therapy. Moreover, with the help of computer modelling, various modifications of the substrate molecules enable to perform structure-activity relationship studies of β-N-acetylhexosaminidases from different sources in vitro as well as in silico.
β-N-Aacetylhexosaminidases vs. modified substrates
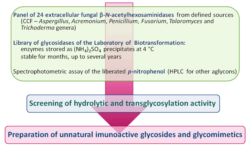
C6 derivatives of pNP-GlcNAc
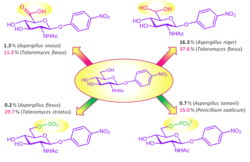
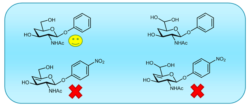
4-Deoxy glycosides as substrates for β-N-acetylhexosaminidases
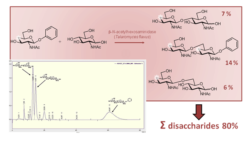
4-Deoxy glycosyl donors in transglycosilation
Reviews
Recent papers
- Slámová et al. Bioorg. Med. Chem. Lett. 2010
- Slámová et al. Glycobiology 2010
- Loft et al. ChemBioChem 2009
- Bojarová et al. J. Mol. Catal. B 2008
Cooperation
- Doc. R. Ettrich, USBE Nové Hrady
- Prof. K. Bezouška, Faculty of Science, Charles University in Prague
- Dr. S. Riva, ICRM-CNR, Milano, Italy
- Prof. L. Elling, RWTH Aachen, Germany


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.